TM3. Counting Electrons in Transition Metal Complexes
Transition metals bind to ligands in order to edge closer to electronic saturation. In other words, the electrons donated by the ligands to form the bonds to the metal help that metal get to the next noble gas configuration. It doesn't always get there, and sometimes it overshoots, but that's a rough guide.
Consider the coordination complex below. [Co(NH3)6]Cl3 contains a cobalt complex ion and three chloride counterions; they are just there to balance the charge. We could think of that complex as being assembled from a cobalt ion and six ammonia molecules. The six ammonia molecules each lend a lone pair to help the cobalt towards its octet. If we go backwards, and disassemble the complex to its components, we see that each of those ammonia molecules was neutral. There is no formal charge on any of those nitrogens. If they are all neutral, but the complex has a positive charge, then where did the positive charge come from?

Figure TM3.1. Deconstructing a coordination complex: showing the pieces it came from.
The answer has to be the cobalt ion. The cobalt has a 3+ charge (or "oxidation state"). Knowing that, we can figure out how many electrons cobalt has in its valence shell within the complex.
In the periodic table, cobalt has nine valence electrons. However, if the overall complex has a charge of 3+, then the cobalt also had a charge of 3+. It has lost three electrons, so it only has six left. Once it forms the complex, each ammonia donates a pair of electrons; that's twelve total. The valence shell around cobalt includes its own d electrons plus that twelve donated by the ligands, for a total of eighteen.
We can summarise in a table:
Table TM3.1. Electron counting in [Co(NH3)6]3+.
| metal valence e- (or d e-) | 9 e- |
| charge on complex | 3+ |
| charge on ligands | 0 |
| charge on metal | 3+ |
| metal ion d e- | 6 e- |
| e- donated by ligands | 6 x 2 = 12 e- |
| total | 18 e- |
- The total number of electrons around the metal in the complex includes the metal ion's valence electrons and the electrons shared by ligands.
- Ligands donate 2 electrons each.
- The number of valence electrons on the metal ion are the number of valence electrons on the metal ion minus the charge on the metal ion.
- The charge on the metal ion is the charge on the complex minus any charges on ligands.
[Co(NH3)5Cl]Cl2 is a very similar example. This time, when we dissect the complex, we find a chlorine that would have a formal charge of 1- (we always assume the ligands all had octets before they joined the metal). The overall complex has a 2- charge; taking into account that there is a charge of -1 on the chloride within that complex, then the cobalt ion must have a charge of 3+ in order to arrive at that overall 2+ charge.
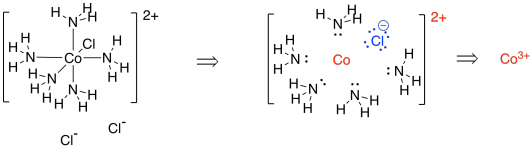
Figure TM3.2. Deconstructing another coordination complex.
Again, we can use a table to count the electrons on cobalt in the complex.
Table TM3.2. Electron counting in [Co(NH3)5Cl]2+.
| metal valence e- (or d e-) | 9 e- |
| charge on complex | 2+ |
| charge on ligands | 1- |
| charge on metal | 3+ |
| metal ion d e- | 6 e- |
| e- donated by ligands | 12 e- |
| total | 18 e- |
